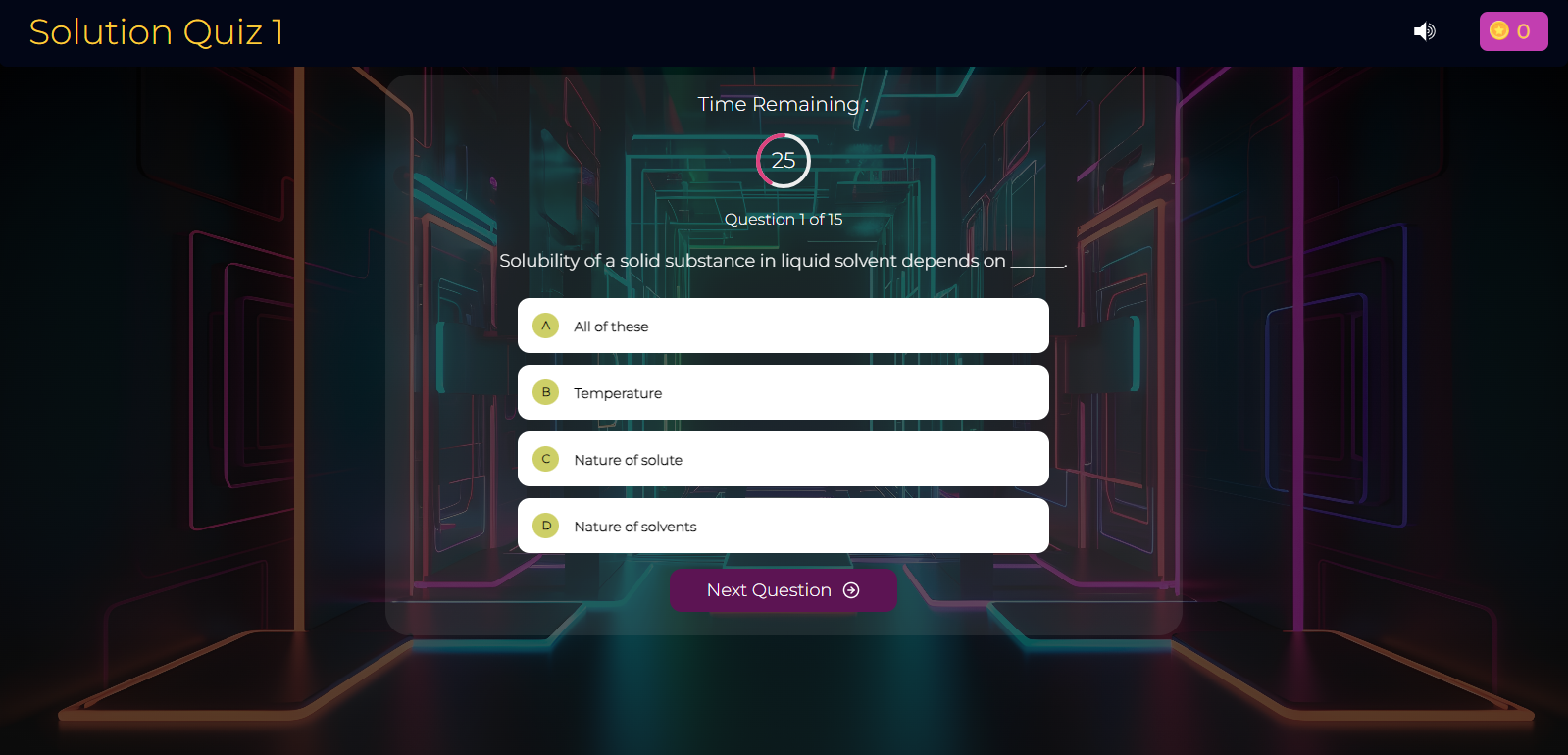
Kinetic Theory of Gases Quiz-2
Popular Questions In Kinetic Theory of Gases Quiz-2
The temperature at which the mean speed of the molecules of hydrogen gas equals the escape speed from the earth is Given escape speed from the earth
One kilogram of a diatomic gas is at a pressure of The density of the gas is 4 kg . The energy of the gas due to its thermal motion is
Two gases are under identical conditions of temperature, pressure, and volume. The ratio of mean free paths of two molecules of gas having molecular diameter 1A and 2A is
A gaseous mixture consists of molecules of types A, B and C with masses Error in LaTeX 'm_A > m_B > m_C ': KaTeX parse error: Expected 'EOF', got '&' at position 5: m_A &̲gt; m_B > m_…. The correct relation between their average kinetic energy is
A balloon has 5.0 gram mole of helium at . The total internal energy of the system is
Air is pumped into a cycle tyre. Which of the following statements is correct?
One mole of an ideal gas (monoatomic) is mixed with one mole of diatomic gas, the ratio of specific heats at constant pressure and at constant volume, for the mixture is
The ratio of vapour densities of two gases at a given pressure in 9:8. The ratio of their rms speed is
Two non-reactive monoatomic ideal gases have their atomic masses in the ratio 2:3. The ratio of their partial pressures, when enclosed in a vessel kept at constant temperature, is 4:3. The ratio of their densities is
An ideal diatomic gas at 300 K has molecules of moment of intertia . The root mean square angular velocity of the molecules in radian per second is