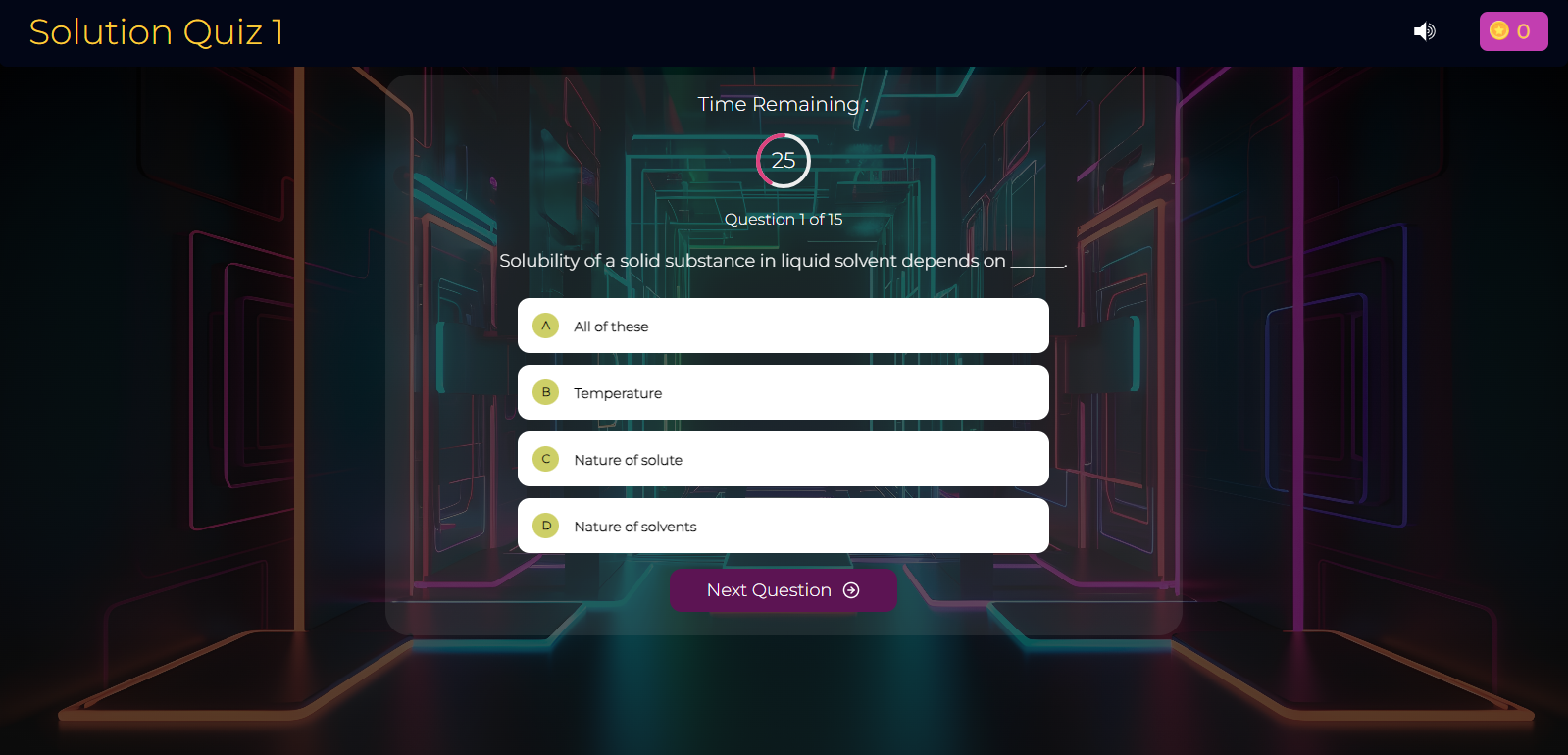
Chemistry: Cracking the Salt Analysis Code
Difficulty Level :
10 Questions
Popular Questions In Chemistry: Cracking the Salt Analysis Code
What is the purpose of performing a 'flame test' in salt analysis?
Which of these gases is typically evolved when a carbonate salt reacts with a dilute acid?
What color flame does Copper (Cu) usually produce in a flame test?
Which reagent is commonly used to test for the presence of chloride ions (Cl⁻) in a salt solution?
A pungent smelling gas, which turns red litmus blue, is likely which of the following?
What does the 'brown ring test' indicate the presence of?
Which of the following salts is insoluble in water?
A white precipitate insoluble in dilute nitric acid indicates the presence of:
What is the color of the precipitate formed when barium chloride (BaCl₂) is added to a solution containing sulfate ions (SO₄²⁻)?
Which cation produces a lilac (pale purple) flame color?